Introduction:
In patients undergoing allogeneic hematopoietic cell transplantation (HCT), venous thromboembolism (VTE) is associated with increased morbidity and worse survival. Central venous catheters (CVC) are placed for administration of both stem cell products and other supportive measures. CVC are a common etiology of VTE during HCT. This study examines the associations between patient- and CVC-related variables and the incidence of catheter-related thrombosis (CRT) in patients undergoing allogeneic HCT.
Methods:
We selected consecutive patients who underwent their first allogeneic HCT from MD Anderson Cancer Center between 2016-2020. The electronic medical records (EMR) of these patients were examined using ICD 9/10 codes, and radiology reports using a natural language processing (NLP) algorithm to look for VTE events. All VTE events were confirmed via manual chart review. Details on the type of CVC, anatomic venous location, laterality, and size were extracted. For this study, CVC was defined as a catheter that extends into the superior vena cava; these catheters were classified as either a peripherally induced central catheter (PICC), non-tunneled CVC, or tunneled CVC.
Unadjusted and multivariable Cox proportional hazards models were used to assess the association between patient and transplant factors and time to CRT. Acute graft versus host disease and active infection were included in the models as categorical time varying covariates. Cumulative incidence competing risk model was used to assessed CRT incidence with death as a competing cause.
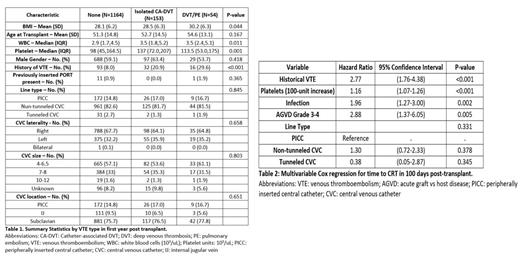
Results:
There were 1371 adult patients included in the study. Most patients had non-tunneled CVC as their central access, with the subclavian vein being the most used location (Table 1). A total of 207 patients had VTE during the first year post-HCT, with 153 having isolated CRT and 54 having other VTE (Table 1). The incidence of CRT was 7.7% at 100-day and 11.3% at 1-year, with the incidence rate at a near constant estimate in the first 100 days. The cumulative incidence of DVT/PE with death as a competing risk factor was 1.17% at 100-day and 4.08% at 1-year. In Cox regression models for CRT by 100 days, history of VTE, higher platelet count, acute GVH disease stage 3-4, and infection were statistically associated with time to CRT (Table 2). The type of CVC (non-tunneled CVC and PICC vs. tunneled CVC) was non-significantly associated with increased risk of CRT.
Conclusion:
In our cohort of patients undergoing allogeneic HCT, the incidence of CRT appeared to be higher than that reported in the literature. Significant risk factors associated with CRT occurred included VTE history, higher pre-transplant platelet count, and development of severe acute GVHD or active infection. While the type of CVC did not reach statistical significance due to the majority of patients receiving non-tunneled CVC as a standard institutional practice, there was a trend of increased CVC in this group of patients. Future multi-center studies that incorporate different types of CVC will further elucidate this potential association with CRT post-transplant.
Disclosures
Kebriaei:Jazz: Consultancy, Honoraria; Pfizer: Consultancy, Honoraria. Shpall:Takeda: Other: License agreement; NY Blood Center: Membership on an entity's Board of Directors or advisory committees; Affimed: Other: License agreement; Adaptimmune: Membership on an entity's Board of Directors or advisory committees; Navan: Membership on an entity's Board of Directors or advisory committees; Celaid Therapeutics: Membership on an entity's Board of Directors or advisory committees; Fibrobiologics: Membership on an entity's Board of Directors or advisory committees; Axio: Membership on an entity's Board of Directors or advisory committees; Syena: Other: License agreement. Rojas Hernandez:Anthos Therapeutics: Research Funding.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal